Millions of prescriptions are compounded by pharmacists, nurses, and physicians each year in the US to meet the unique needs of patients. Compounding provides access to medication for patients who may not be able to use commercially available formulations due to dosing requirements, allergies or rare diseases.
Compounding plays a critical role in:
- Situations where strength or dosage of a medicine needs to be customized
- Reformulating a drug to exclude nonessential ingredients such as dyes to which a patient may be allergic
- Changing the dosage form of the medication for patients who may have difficulty swallowing
- The treatment of rare diseases
- Clinical trials
Compounded drugs made without the guidance of standard procedures or practices may be inconsistent, sub-potent, super potent or contaminated, exposing patients to significant risk of adverse events or even death.
USP Compounded Preparation Monographs
USP Compounded Preparation Monographs contain formulations and procedures for practitioners who are compounding specific preparations where a commercially available product is not available to meet the individual health needs of a patient. Using the compounded preparation monograph will help the compounder produce a preparation of consistent identity, strength and potency.
USP Compounded Preparation Monographs provide standard instructions for the following:
- Formulas (ingredients and quantities)
- Directions to correctly compound the preparation
- Beyond-use dates based on stability studies
- Packaging and storage information
- Acceptable pH ranges
- Stability-indicating assays
Donate Today
USP requests submissions of compounded formulations and supporting scientific information to create Compounded Preparation Monographs that address health needs of vulnerable populations such as pediatrics and geriatrics.
List of Compounded Preparation Monographs
The donated information will be used to establish formulas, preparation procedures, assays, testing methods and stability criteria required to develop Compounded Preparation Monographs. The USP Compounding Expert Committee is responsible for developing and revising Compounded Preparation Monographs based on the data submitted.
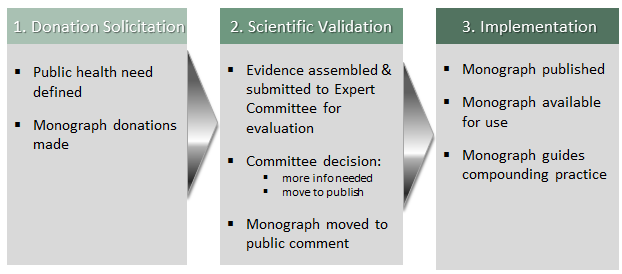
Creating a Compounded Preparation Monograph is a three step process:
Eligible Donors of information for Compounded Preparation Monographs
What information should be submitted?
|
Who is eligible to submit?
|
Benefits of Participating in the Compounded Preparation Monograph Donation Program
Promote Public Health
By participating in this program, you will promote public health by contributing to the creation of a comprehensive repository of compounded preparation monographs in the USP–NF.
Donor Recognition Program
USP values its donors and volunteers in the monograph development process, and recognizes these contributions with a certificate of appreciation. USP also acknowledges significant scientific contributions and participation in the process in a variety of ways, including the following:
- Recognition in USP publications
- Recognition on USP’s annual donor list
- Personalized engraved crystal globe
recognition statue - Complimentary workshop registrations or
Pharmacopeial Education and Training courses - Complimentary print subscription to one of USP’s publications
- Complimentary USP Reference Standards
Donate Today: Contact CPMP Program Manager
Resources
- Download the Compounded Preparation Monograph Donation Program Fact Sheet
- For additional information about the benefits of donating to USP, read the Donor Recognition Fact Sheet.
- Sign up for the Healthcare Quality Standards Newsletter to receive updates on USP Compounding Standards.















